The Danish Medicines Council recently unveiled its 2023 Annual Report, shedding light on the evolving landscape of Health Technology Assessment (HTA) in Denmark. This report not only provides valuable insights but also has significant implications for pharmaceutical spending in the country.
Applications and Evaluations
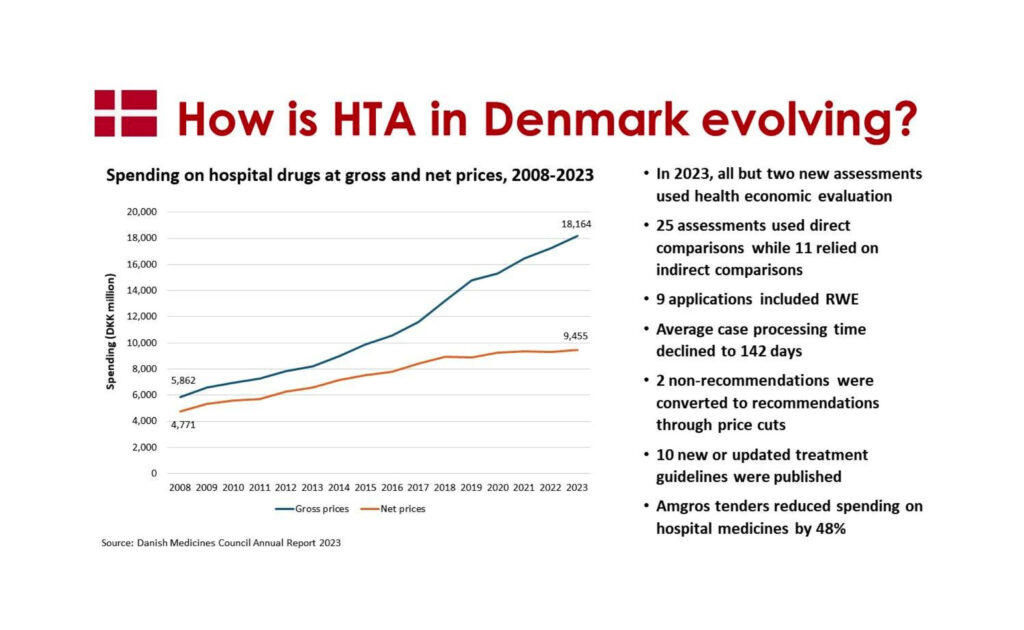
In the past year, the Council received a total of 57 applications for the assessment of new drugs or indications. These applications comprised 38 new evaluations and 19 reassessments based on either new data or revised pricing structures. Notably, nearly all new assessments incorporated health economic evaluations to inform decision-making.
Clinical Trials and Patient Populations
The majority of applications were grounded in evidence from single clinical trials. On average, these studies involved 686 patients, with the highest trial enrolling 5,591 patients and the smallest involving just 35 patients. Such reliance on single trials underscores the need for robust and comprehensive data.
Regarding the source of evidence, 33 out of 36 assessments drew from data obtained during Phase III trials. However, two applications utilized information from Phase I or II studies, and one assessment exclusively relied on a Phase I trial. This diversity in data sources ensures a holistic evaluation of drug efficacy and safety.
Comparisons and Real-World Evidence
In assessing new treatments, 25 out of 36 evaluations directly compared them with existing therapies. The remaining 11 assessments relied on indirect treatment comparisons. This approach allows the Council to gauge relative effectiveness and make informed recommendations.
Furthermore, nine out of 36 applications incorporated real-world evidence—data collected from actual clinical practice settings. This inclusion enhances the Council’s ability to understand how drugs perform in real-world scenarios beyond controlled clinical trials.
Efficiency and Decision-Making
Efforts to streamline the assessment process have yielded positive results. In 2023, 56% of applications were completed within the 16-week deadline (from submission of an adequate application), a significant improvement compared to the previous year’s 24%. The average case processing time also decreased from 22 weeks in 2022 to 20 weeks and 2 days in 2023. These efficiency gains allow for more timely decisions and better patient access to innovative therapies.
Price Negotiations and Recommendations
When faced with high drug prices, the Council takes an active role in encouraging manufacturers to adjust their pricing. Since its establishment in 2017, the Council has successfully transformed 12 non-recommendations into recommendations through price reductions. Notably, in 2023, two drugs—Pegcetacoplan and inclisiran—received favorable recommendations following price adjustments. Conversely, a price reduction also influenced the Council’s stance on tucatinib.
In summary, the Danish Medicines Council’s 2023 Annual Report highlights the dynamic landscape of HTA in Denmark. By balancing evidence-based evaluations, real-world data, and efficient decision-making, the Council plays a pivotal role in shaping pharmaceutical access and affordability in the country.
