The Innovative Medicines Fund (IMF) represents a significant stride in healthcare accessibility, stemming from the triumph of its predecessor, the Cancer Drugs Fund (CDF). Launched in June 2022, the IMF extends its reach beyond cancer medications, encompassing any non-cancer drugs endorsed by the National Institute for Health and Care Excellence (NICE) under a regimen of managed access.
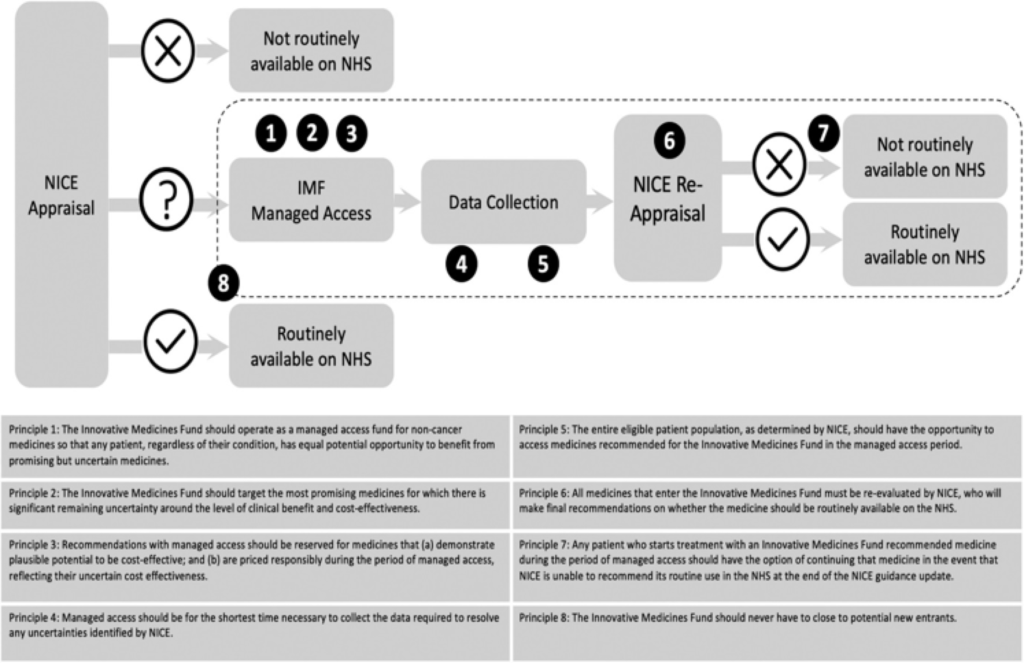
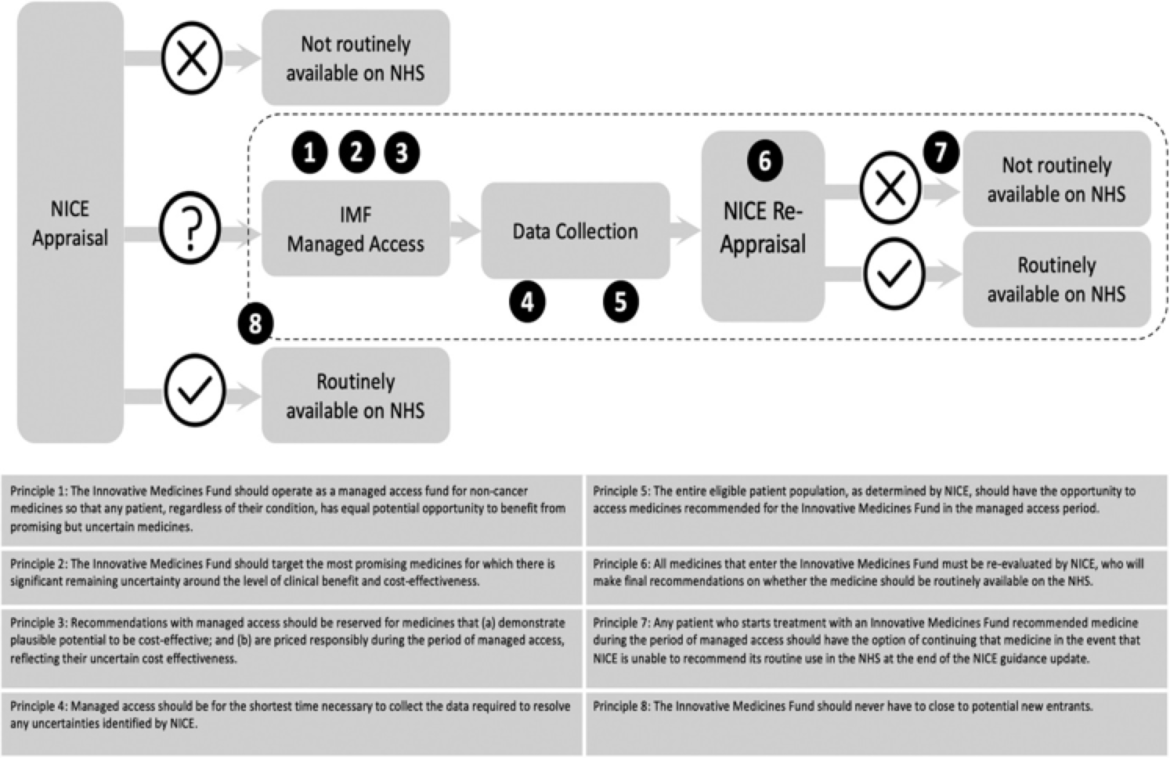
Figure 1. The IMF process and its guiding principles. Sources: Adapted based on NHS England Innovative Medicines Fund Principles and NHS Commercial Framework for New Medicines. NICE: National Institute for Health and Care Excellence; NHS: National Health Service; IMF: Innovative Medicines Fund.
In the landscape of healthcare, accessibility to groundbreaking medications stands as a beacon of hope for countless patients. However, the journey from development to patient access is fraught with challenges, particularly concerning the rising costs of new drugs and the need for careful resource allocation. In this regard, the Innovative Medicines Fund (IMF) emerges as a pivotal solution, building upon the successes of its predecessor, the Cancer Drugs Fund (CDF), to extend access beyond cancer treatments.
The Rising Need for Accessible Healthcare:
The escalating prices of novel pharmaceuticals underscore the necessity for stringent resource allocation strategies within healthcare systems. Health technology assessment (HTA) serves as a crucial tool in navigating these complexities, offering insights into the clinical benefits and cost-effectiveness of new drugs. However, the lower evidentiary requirements for regulatory approvals have introduced uncertainties in reimbursement decisions, prompting the establishment of the IMF.
Navigating Challenges:
The IMF represents a paradigm shift in healthcare accessibility, operating under the ethos of managed access to expedite patient access to potentially transformative medications. Yet, challenges persist regarding its operationalization and the ethical imperative of equitable access across disease areas. Robust evidence generation and safeguards against financial loss emerge as critical considerations in maximizing the IMF’s impact.
Principles Guiding the IMF:
Aligned with the principles outlined by NHS England, the IMF operates with a focus on equitable access, responsible pricing, and rigorous evaluation of medications. Entry into the IMF necessitates early engagement with regulatory bodies, with companies required to demonstrate the potential clinical benefit and address uncertainties through evidence generation.
Ethical Considerations:
Ensuring equal access across disease areas remains a cornerstone of the IMF’s mission, emphasizing the importance of transparency and public scrutiny. While expanding access beyond cancer treatments is commendable, careful consideration must be given to balancing priorities and upholding ethical standards.
Addressing Challenges:
Challenges in evidence generation and the risk of incentivizing high-priced drugs with weak evidence underscore the need for strict guidelines and transparency measures. Acknowledging the opportunity cost of IMF funding is essential in prioritizing cost-effective care for overall population health.
The Innovative Medicines Fund (IMF) has expanded its coverage in England, now encompassing three groundbreaking drugs. Among the latest additions to benefit from the £340 million fund are Alexion’s Kanuma (sebelipase alfa) for the rare genetic disorder Wolman disease, Novartis’ Cosentyx (secukinumab) for hidradenitis suppurativa, and Gilead’s Hepcludex (bulevirtide) for chronic hepatitis D. This expansion, initiated in June 2022, marks a significant stride in facilitating access to innovative treatments with substantial clinical promise.
According to NHS England (NHSE), the primary objective of the Innovative Medicines Fund is to serve as a managed access fund, providing early access to medications while uncertainties in evidence are resolved. Under these provisions, funding could be accelerated by up to five months, commencing from the issuance of draft positive final guidance by the National Institute for Health and Care Excellence (NICE).
However, managed access (coverage with evidence development) is not obligatory for IMF inclusion. NHSE disclosed that sebelipase alfa would not undergo managed access but would receive immediate funding from the IMF, enabling routine patient access up to five months earlier than anticipated.
This arrangement may come as unexpected. NICE’s final draft guidance on Kanuma indicates uncertainties surrounding its clinical effectiveness and cost-effectiveness, prompting questions about its long-term impact on patient outcomes. Nevertheless, Alexion has established a commercial arrangement with NHSE, facilitating patient access through a simple discount patient access scheme.
Similarly, for Cosentyx, Novartis proposed consideration by NICE only for individuals unable to use adalimumab or those experiencing inadequate response to it. While a complex patient access scheme is in place for secukinumab, neither NICE nor NHSE has mentioned managed access.
Limited information is publicly available regarding the terms of Hepcludex’s inclusion in the IMF. NICE’s appraisal acknowledged Gilead’s positioning of the drug for patients with treatment-resistant chronic hepatitis D, despite uncertainties regarding its duration of efficacy. A simple discount patient access scheme has been established for bulevirtide, although managed access is not mentioned.
Overall, the expansion of the Innovative Medicines Fund to encompass these groundbreaking drugs underscores efforts to streamline access to innovative treatments, albeit with nuances in the implementation of managed access provisions.
Conclusion:
The IMF represents a beacon of hope in the realm of healthcare accessibility, offering a pathway to expedite patient access to transformative medications. However, its success hinges on robust evidence generation, transparency, and ethical considerations. By navigating challenges and upholding principles of equitable access, the IMF holds the potential to transform patient care and optimize population health outcomes.
1. National Institute for Health and Care Excellence (NICE) – [Official Website](https://www.nice.org.uk/)
2. NHS England – [Official Website](https://www.england.nhs.uk/)
3. “The Innovative Medicines Fund: a universal model for faster and fairer access to new promising medicines or a Trojan horse?” by Aris Angelis et al. – [Journal Article](https://journals.sagepub.com/doi/epub/10.1177/01410768231192476)
4. NHS England Innovative Medicines Fund Principles – [Official Document](https://www.england.nhs.uk/wp-content/uploads/2022/06/innovative-medicines-fund-principles-v1.1.pdf)
5. NHS Commercial Framework for New Medicines – [Official Document](https://www.england.nhs.uk/wp-content/uploads/2022/06/innovative-medicines-fund-commercial-framework-v1.1.pdf)