We have been using QALYS since I was 18 since it was vigorously defended by Alan Williams In late March, the House Committee on Energy and Commerce made significant strides by advancing H.R. 485, known as the Protecting Health Care for All Patients Act. However, the vote fell along party lines, with Republicans favouring an amended version of the bill. This amended version aims to curtail the use of comparative effectiveness research measures, both in the public and private sectors, particularly in assessing the value of medications for patients. Primarily initiated by Congresswoman Cathy McMorris Rodgers
I personally have reservations about employing incremental cost-per-QALY ratios for comparing treatments, especially when dealing with diseases that exhibit significant qualitative differences. In my view, constructive criticism is essential, and I firmly oppose any restrictions on concepts such as the QALY.
However the debate surrounding QALYs is not new. In the late 1980s and early 1990s, Britain witnessed a robust discourse on the topic, with respected scholars offering contrasting views. Despite criticisms, the QALY remains a pivotal tool in assessing the value of medical interventions.
While acknowledging some flaws in the QALY approach, it’s essential to remain constructive in criticism. Outright bans on concepts like the QALY may hinder progress without offering viable alternatives. In allocating finite healthcare resources, a method to measure the value of medical interventions remains crucial, and the QALY serves this purpose, albeit imperfectly.
Critics often target the use of incremental cost-per-QALY thresholds for reimbursement decisions. However, setting these thresholds has been subject to criticism due to their perceived arbitrariness. In England and Scotland , for example, the threshold value has remained unchanged since 1999, raising questions about its relevance in contemporary healthcare contexts.
While the QALY is not without its flaws, it provides a framework for comparative cost-effectiveness research, aiding policymakers in maximizing health outcomes within budgetary constraints. Policymakers must consider a range of factors beyond just QALY analyses when making decisions, including the availability of alternative therapies and non-quantifiable benefits and risks of care.
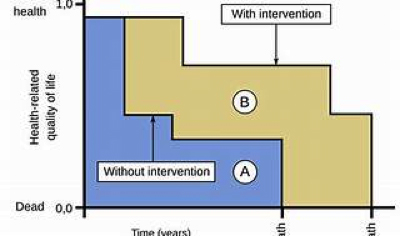
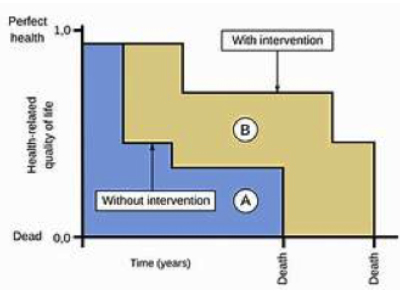
The Quality-Adjusted Life Year (QALY) is a conceptual framework that exhibits flexibility, allowing it to address certain concerns raised by critics. Notably, it can consider the severity of disease. For instance, adjustments can be made by incorporating weights related to disease burden. Additionally, an alternative approach exists—one that assigns equal value to additional years of life across different diseases and populations. This approach is known as the Equal Value Life-Year Gained (evLYG).
The QALY can be used in comparative cost-effectiveness research to inform decisions about maximizing people’s health given limited budgets, because this measure allows for comparison of health gains across treatments and diseases.
Ultimately, policymakers must be equipped with evidence-based tools to make informed decisions that optimize healthcare outcomes for the population. Without alternatives that are practical and effective, rejecting the QALY risks perpetuating ad-hoc decision-making that may not be equitable or evidence-based.
The impact of not using quality-adjusted life years (QALYs) in U.S. healthcare decision-making can be multifaceted and could influence various aspects of the healthcare system:
1. Resource Allocation: Without a standardized metric like QALYs, decision-makers may face challenges in comparing the cost-effectiveness of different healthcare interventions. This could lead to inconsistencies in resource allocation and potentially result in suboptimal use of healthcare resources.
2. Treatment Coverage and Reimbursement: QALYs are often used by insurers and healthcare payers to determine coverage and reimbursement for medical treatments. Without QALYs, payers may rely on other criteria or subjective judgments, potentially leading to disparities in access to care or coverage for certain treatments.
3. Research and Development: QALYs play a role in health economics research and modelling, including assessments of the cost-effectiveness of new drugs and interventions. The absence of QALYs could affect the evaluation of new treatments, potentially impacting investment in research and development.
4. Patient Outcomes: QALYs provide a framework for considering both the quantity and quality of life gained from healthcare interventions. Without QALYs, decision-makers may focus solely on clinical outcomes or cost considerations, potentially overlooking broader aspects of patient well-being and quality of life.
5. Policy Development: The use of QALYs can inform healthcare policy decisions at both the federal and state levels. Without a standardized metric like QALYs, policymakers may face challenges in evaluating the potential impact of proposed policies on population health and healthcare costs.
Overall, the impact of not using QALYs in U.S. healthcare decision-making could affect the efficiency, equity, and effectiveness of the healthcare system. It may lead to challenges in allocating resources effectively, determining coverage and reimbursement policies, evaluating the value of healthcare interventions, and informing evidence-based policymaking.
The FDA will continue to function without relying on quality-adjusted life years (QALYs) by utilizing alternative methods and criteria for evaluating medical products. Here’s how the FDA can function effectively without QALYs:
1. Clinical Trial Data: The FDA will continue to assess the safety and efficacy of medical products based on rigorous clinical trial data provided by manufacturers. This includes evaluating endpoints such as improvements in symptoms, survival rates, and other clinically meaningful outcomes.
2. Risk-Benefit Analysis: The FDA will conduct risk-benefit analyses to assess whether the potential benefits of a medical product outweigh its potential risks. This analysis takes into account factors such as the severity of the condition being treated, the availability of alternative treatments, and the magnitude of potential benefits and risks.
3. Patient Input: The FDA may incorporate patient input into its regulatory decision-making process to better understand patient preferences and perspectives. Patient input can provide valuable insights into the perceived benefits and risks of treatments and help inform regulatory decision-making.
4. Health Economic Analyses: While QALYs may not be used, the FDA may still consider health economic analyses as part of the regulatory review process to provide additional context on the potential cost-effectiveness of new treatments. These analyses may use alternative metrics or approaches to assess the value of medical interventions.
5. Clinical Guidelines and Standards: The FDA may rely on clinical guidelines and standards developed by professional medical societies and other organizations to inform its regulatory decision-making process. These guidelines often provide recommendations based on clinical evidence and expert consensus.
Overall, the FDA will continue to function effectively by utilizing a combination of clinical data, risk-benefit analyses, patient input, health economic analyses, and clinical guidelines to evaluate the safety, efficacy, and value of medical products. While QALYs may offer one approach to assessing the value of healthcare interventions, their absence does not preclude the FDA from fulfilling its regulatory responsibilities.
